comparing vaccines: efficacy, safety, side effects
there are now four vaccines available to canadians. here's how they differ
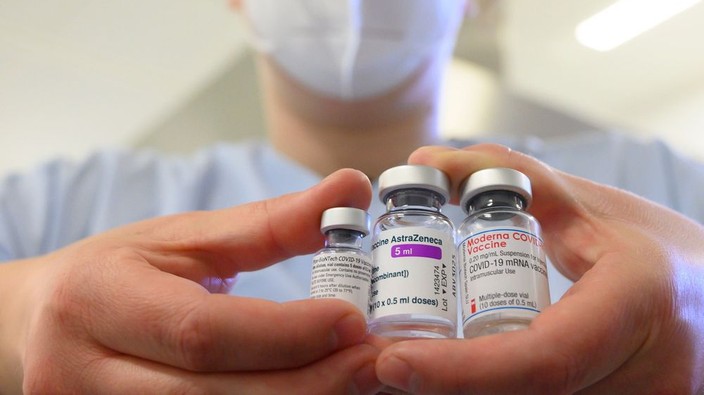
a woman wearing a face mask shows three vials with different vaccines against covid-19 by (l-r) pfizer-biontech, astrazeneca and moderna.
thomas kienzle/afp via getty images
/
afp via getty images
as more and more canadians become eligible for vaccination in what has become a crowded landscape of covid-19 vaccines, many may wonder what to expect when they roll up their sleeves. there are now four vaccines available to canadians. astrazeneca and the university of oxford’s vaccine as well as the first single-dose shot from janssen, a unit of johnson & johnson, were recently authorized by health canada, joining the pfizer-biontech and moderna mrna vaccines approved in december.how do the four vaccines compare in efficacy and safety?the pfizer and moderna vaccines have the highest efficacy at around 95 per cent. lower efficacy, in the 60 to 70 per cent range, was found for the astrazeneca and janssen products. (efficacy measures how many vaccinated people contract covid-19 compared with how many infections occur in the placebo or control group. put another way, it represents the proportion of covid-19 infections that could be prevented by vaccination).side effects are common in all four. they appear to be much the same as some vaccines routinely recommended for older adults like the shingles vaccine, but worse than other vaccines like the high-dose flu shot. in the astrazeneca trial, in which the control group received a meningococcal vaccine rather than a placebo, a similar proportion of younger participants experienced reactions to the meningococcal vaccine and the covid-19 vaccine.older adults appear to have fewer reactions that affect the whole body, such as fevers, while enjoying similar or even higher protection than younger adults.however, we should be cautious in comparing these trials since they used different ways to measure efficacy, says alison thompson, an associate professor who studies public health policy and ethics at the university of toronto. “i think the johnson and johnson (janssen) one had much better clinical endpoints in the trials … there was a pretty low bar for establishing efficacy in the other trials.”the pfizer and moderna trials looked at prevention of any lab-confirmed infection starting at least seven to 14 days after the last vaccine dose in those who had symptoms. however, the janssen trial set a higher bar, looking to prevent moderate to severe or critical infection. “as we get more data about the kind of efficacy that they have over the longer term, we may see those (efficacy) numbers come down significantly for the mrna (pfizer and moderna) vaccines,” says thompson.the lower efficacy in the astrazeneca and janssen trials may also be explained by a greater number of infections from variants of concern, which emerged after the pfizer and moderna trials were completed and against which vaccine protection is expected to be lower. in the janssen trial, for example, efficacy was 74 per cent in the united states but dropped to 52 per cent in south africa, where almost 95 per cent of cases were due to the b.1.351 variant. at least 136 cases of this variant had been detected in canada as of march 8.comparisons should also take into account differences in the number and timing of doses. however, the national advisory committee on immunization expects that short-term efficacy will likely remain high, even with an extended four-month interval between doses.how well do these vaccines work in different populations?the vaccines appear to work equally well across subgroups defined by age, sex, race, ethnicity and among those at risk for developing severe covid-19. all four seem to protect against more severe cases and hospitalization, but evidence is still limited.importantly, all four vaccines exceed health canada’s 50 per cent efficacy standard. “while each of the vaccines that health canada has authorized has different efficacy numbers, the reality is that you will have a greatly reduced chance of getting covid-19 with any of the vaccines that have been authorized,” supriya sharma, health canada’s chief medical adviser, said in a press conference on march 5. “i would not hesitate to roll up my sleeve to get any of (them).”the national advisory committee on immunization advised in early march against using the astrazeneca shot in adults aged 65 years and older, citing a lack of efficacy data for that age group. as more data comes in, “we’ll be able to make better decisions about who ought to get which vaccines,” thompson predicts.what are the expected side effects?just over half of recipients in the clinical trials for all four vaccines experienced at least one reaction affecting their entire body, like fatigue or muscle aches, after their first dose; this increased to 60 to 80 per cent after the second dose.side effects were more common after the second shot of the pfizer and moderna mrna vaccines, while astrazeneca recipients reported them more frequently after the first dose.most symptoms were mild to moderate and disappeared after a few days. severe reactions – defined as those that prevent daily activities or require medical attention – were rare among all trial participants. most of the side effects are typical for any inoculation. “a little bit of myalgia, fatigue, headache and then injection site reactions, like localized redness, tenderness or swelling are common with all vaccines,” says lacey robinson, an allergist and immunologist at massachusetts general hospital in boston.nonetheless, some frontline health-care providers, who were first in line to receive covid-19 vaccines, say their reactions were enough to miss a day of work, particularly after the second shot of the pfizer or moderna vaccines. “i had mine last night and i’m melting into the couch,” said yuliya rackal, a family doctor in toronto, after getting her first pfizer shot.for others, however, the reaction has been much milder. “really painless,” said karen swirsky, a family doctor at st. michael’s hospital in toronto, though she adds that she took pain medication for a sore arm after her second pfizer jab. “very similar to what i would get with a seasonal flu shot,” said marvin gelkopf, another toronto-area family physician, who described his experience of the shingrix (shingles) vaccine as “way worse than this.”side effects are also more common in younger adults, who make up the bulk of essential workers.the frequency and type of reaction may depend on which vaccine you receive. “we certainly are seeing more delayed injection site reactions with the moderna vaccine, which was also seen in the trials, more commonly about a week after vaccination,” says robinson. however, she adds, “it probably is a great sign that you’re mounting a really good immune response to the vaccine.”for those who do experience side effects, physicians recommend at-home treatment like tylenol or motrin for pain relief or ice on the arm. generally the reactions will go away after a few days. “most people are feeling back to normal very quickly,” says robinson.allergic reactions like anaphylaxis are rare but treatable complications. they occur most often in people with a history of allergic reactions.other differencesthe four vaccines use slightly different ways to trick the immune system into producing a response. the pfizer and moderna vaccines use messenger rna, or mrna, to deliver genetic instructions to the host’s immune cells to build copies of the viral spike protein. astrazeneca and janssen hijack another respiratory virus, known as adenovirus, to deliver these instructions.both the astrazeneca and janssen products can be stored in refrigerators while the pfizer and moderna mrna vaccines, which are less stable at those temperatures, must be stored in freezers. this has already raised issues of equitable access. because of extreme temperature storage requirements, the pfizer and moderna vaccines can only be administered in settings with the necessary freezers, like hospital clinics in major urban centres.the bottom line is that most canadians will not have a choice of vaccines due to limited supply.“our advice to canadians is to get whichever vaccine is available to you. the longer you wait to get vaccinated, the longer the time goes by that you are not protected,” says sharma.as of march 8, canada had administered a total of 2.5 million doses. across canada, almost two million people have gotten at least one dose and more than 579,000 people, or about 2 per cent of the adult population, are fully vaccinated. canada has signed agreements for up to 180 million doses in total with manufacturers of the four authorized vaccines: up to 76 million doses for pfizer, 44 million for moderna, 22 million for astrazeneca and up to 38 million for janssen.catharine chambers is a phd candidate in epidemiology and vanier scholar at the dalla lana school of public health at the university of toronto.
disclaimer: differences in study population, eligibility criteria and study protocols should be considered when comparing data across clinical trials.
this story was originally published on healthy debate. find the original story here.
 6 minute read
6 minute read





