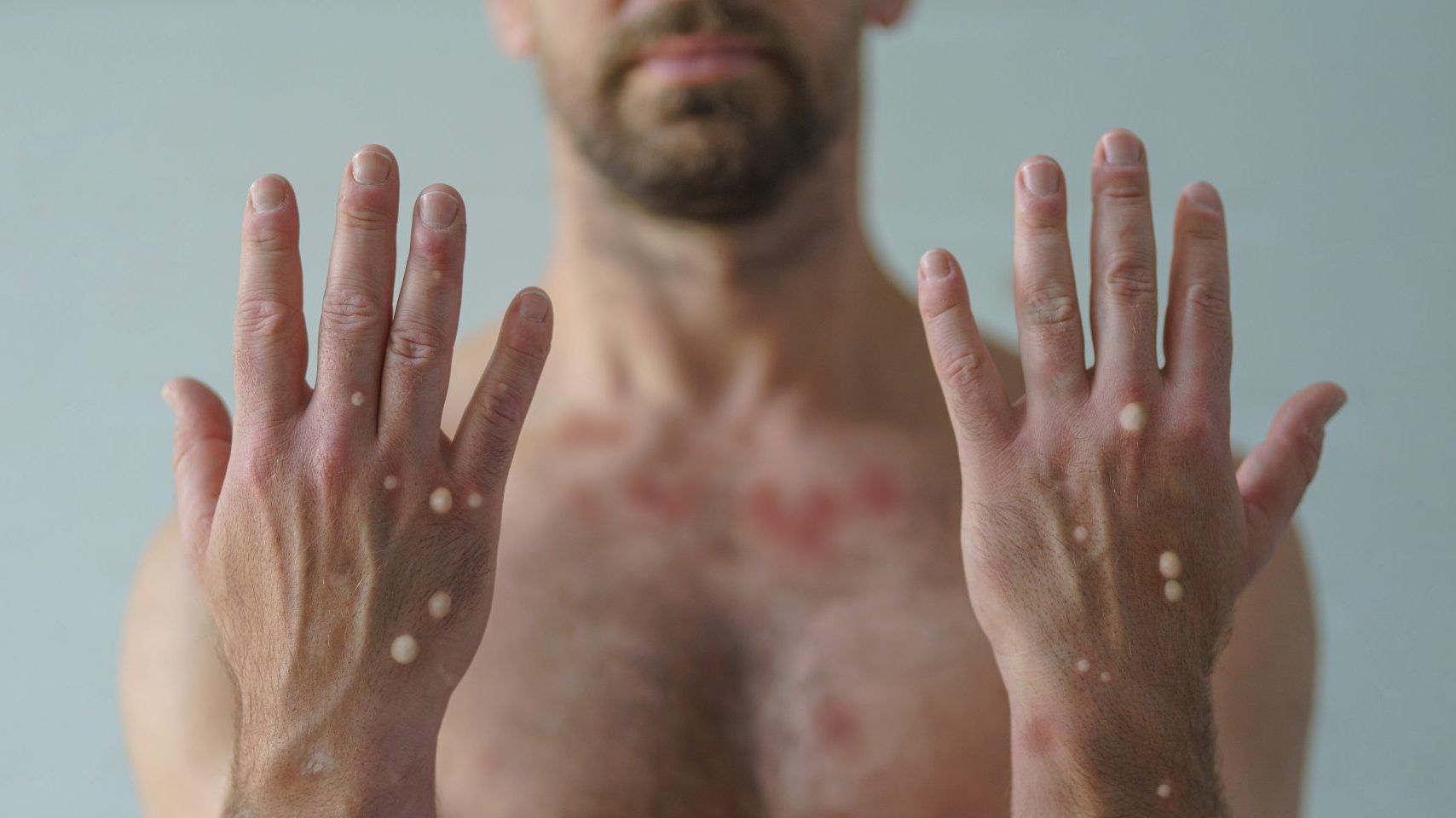
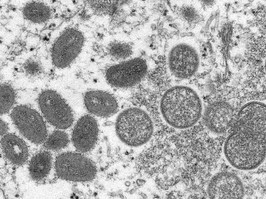
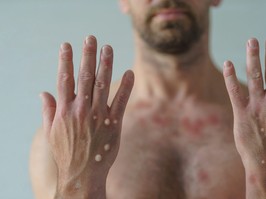
a second person has died in the u.s. after contracting monkeypox; officials
there are currently two deaths under investigation in the u.s. for a possible link to the rare infection.
monkeypox: experts at aids conference say the current global response is not enough
there are now more than 800 confirmed cases of monkeypox in the country.
 2 minute read
2 minute read