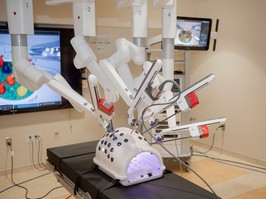
pigs are 'the solar and wind of organ availability'
the successful transplant of a pig's kidney in a human signals a major step forward in the life-saving use of animal organs in people.
depression may make people more likely to believe covid misinformation
they found that people with depression were 2.2 times more likely to endorse misinformation about the vaccines.
 4 minute read
4 minute read